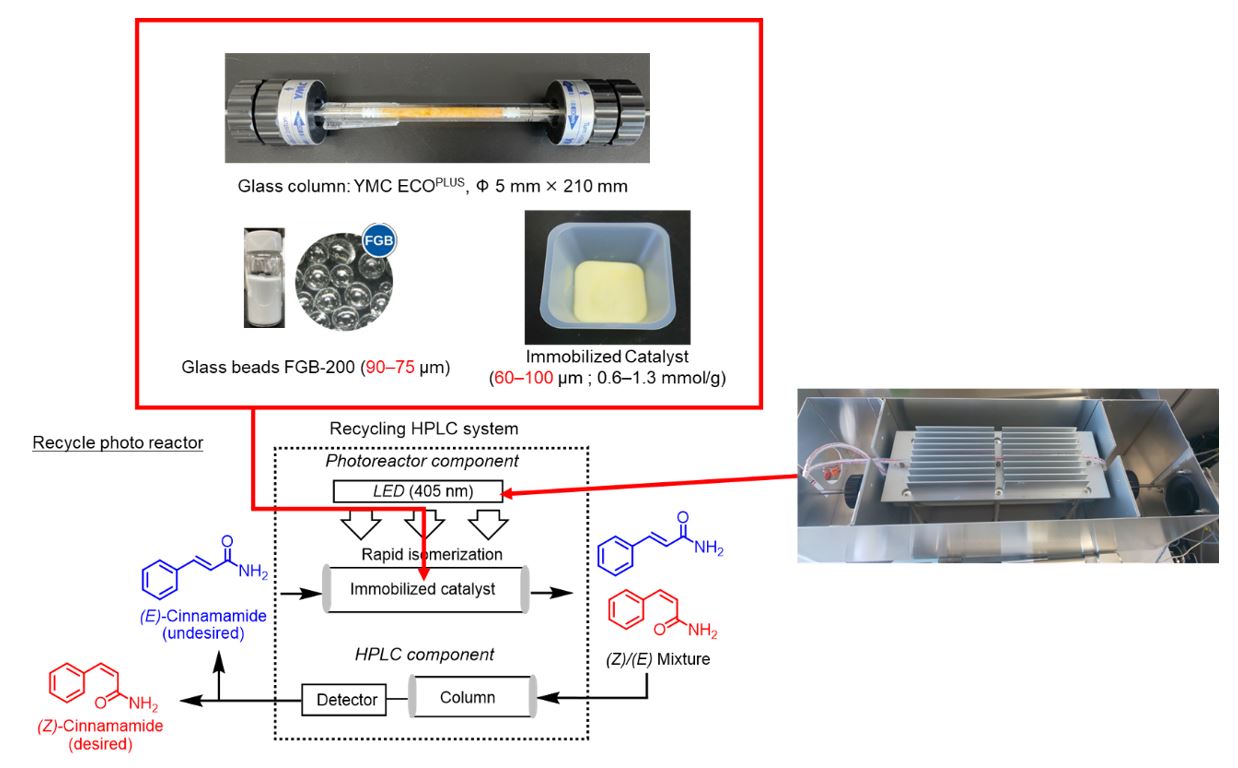
Dendritic structures that emerge during the growth of thin films are a major obstacle in large-area fabrication, a key step towards commercialization. However, current methods of studying dendrites involve crude visual inspection and subjective analysis. Moreover, growth optimization methods for controlling dendrite formation require extensive trial and error. Now, researchers have developed a new AI model that incorporates topology analysis and free energy to reveal the specific conditions and mechanisms that drive dendrite branching.
Thin film devices, composed of layers of materials a few nanometers thick, play an important role in various technologies, from semiconductors to communication technologies. For instance, graphene and hexagonal-boron nitride (h-BN) multilayer thin films, deposited on copper substrates, are promising materials for next-generation high-speed communications systems. Thin films are grown by depositing tiny layers of materials onto a substrate. The growth process conditions significantly influence the microstructure of these films, which in turn influences their function and performance.
Dendritic structures, or tree-like branching patterns that emerge during growth, pose a major challenge to large-area fabrication of thin-film devices, a key step toward commercial application. They are commonly observed in materials like copper, graphene, and borophene, particularly in the early growth stage and multilayer films. Since the microstructure directly impacts device performance, reducing dendritic formation is, therefore, critical. However, methods for studying dendrites have largely relied on crude visual analysis and subjective interpretation. Understanding the conditions that drive dendritic branching is essential for optimizing the thin-film growth process, but existing approaches often require considerable trial and error.
To address these challenges, a research team, led by Professor Masato Kotsugi from the Department of Material Science and Technology at Tokyo University of Science (TUS), Japan, developed an innovative explainable artificial intelligence (AI) model for analyzing dendritic structures. The team included Misato Tone, also from TUS, and Ippei Obayashi from Okayama University. The team developed a novel method that bridges structure and process in dendritic growth by integrating persistent homology and machine learning with energy analysis. “Our approach provides new insights into growth mechanisms and offers a powerful, data-driven pathway for optimizing thin-film fabrication,” explains Prof. Kotsugi. Their study was published online in Science and Technology of Advanced Materials: Methods on March 7, 2025.
To analyze the morphology of dendrite structures, the team used a cutting-edge topology method called persistent homology (PH). PH enables multiscale analysis of holes and connections within geometric structures, capturing the complex topological features of the tree-like dendrite microstructures that conventional image processing techniques often overlook.
The researchers combined PH with principal component analysis (PCA), a machine learning technique. Through PCA, the essential features of the dendrite morphology extracted via PH were reduced to a two-dimensional space. This enabled the team to quantify structural changes in dendrites and establish a relationship between these changes and Gibbs free energy, or the energy available in a material that influences how dendrites form during crystal growth. By analyzing this relationship, they uncovered the specific conditions and hidden growth mechanisms that influence dendritic branching. Prof. Kotsugi explains, “Our framework quantitatively maps dendritic morphology to Gibbs free energy variations, revealing energy gradients that drive branching behavior.”
To validate their approach, the researchers studied dendrite growth in a hexagonal copper substrate and compared their results with data from phase-field simulations.
“By integrating topology and free energy, our method offers a versatile approach to material analysis. Through this integration, we can establish a hierarchical connection between atomic-scale microstructures and macroscopic functionalities across a wide range of materials, paving the way for future advancements in material science,” remarks Prof. Kotsugi. “Importantly, our method could lead to the development of high-quality thin-film devices leading to high-speed communication beyond 5G.”
This study’s framework could pave the way for breakthroughs in sensor technology, nonequilibrium physics, and high-performance materials by uncovering hidden structure-function relationships and advancing complex system analysis.



.jpeg)