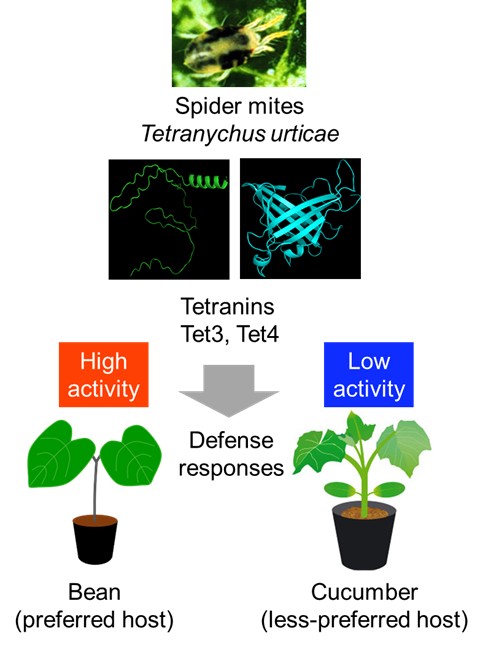
Overuse of chemical pesticides has driven resistance in agricultural pests, including the adaptable two-spotted spider mite. Researchers from Japan have discovered novel elicitor proteins, Tet3 and Tet4, in mite saliva that could enhance sustainable pest control. They found that these proteins play a crucial role in modulating plant defense responses by acting as key players in the complex interactions between parasite and host, paving the way for new mite countermeasures.
As global food demand continues to increase, effective pest control remains one of agriculture’s most pressing challenges. Worldwide, farmers apply nearly 4 million tons of chemical pesticides annually to protect their crops, representing a $60 billion industry. While these compounds have significantly boosted agricultural productivity, their widespread use has raised concerns regarding environmental impact, health risks, and the long-term sustainability of modern farming.
The two-spotted spider mite, Tetranychus urticae, exemplifies the limitations of conventional pesticide-based pest management in agriculture and horticulture. These microscopic arachnids infest a wide range of crops and fruit trees and can reproduce extremely quickly. More importantly, unlike many other pests, they rapidly develop resistance to chemical pesticides, making control efforts increasingly challenging. With pesticide resistance on the rise, farmers worldwide are urgently seeking alternative, sustainable pest control strategies.
A research team led by Professor Gen-ichiro Arimura from the Department of Biological Science and Technology, Faculty of Advanced Engineering, Tokyo University of Science, Japan, closely examined the fine molecular interplay that occurs between T. urticae mites and their host plants. Their study was published online in The Plant Journal on March 4, 2025. The team focused on specific substances called elicitors, secreted by T. urticae, and examined their biological effects on various crops.
“An elicitor is a molecule that plants or pests possess that can enhance the defense response of plants,” explains Prof. Arimura. “In our previous research, we identified two tetranins, labeled Tet1 and Tet2, as elicitors in the salivary glands of two-spotted spider mites; these substances induce defense responses in the common bean and other commercially important crops.”
The research team investigated the effects of an additional 18 salivary gland proteins on the resistance of common bean leaves to T. urticae. According to this initial screening, they identified two new tetranins—Tet3 and Tet4—that appear to reduce the reproduction of spider mites on the plants.
After a series of experiments involving genetic engineering and advanced molecular and biochemical methods, the team uncovered the roles of Tet3 and Tet4 in the complex interactions between T. urticae and its host plants. Interestingly, they found that the expression of Tet3 and Tet4 varies greatly depending on which plant the mites fed on. Mites feeding on common beans, their preferred host, had significantly higher levels of Tet3 and Tet4 expression than those on cucumbers, a less preferred option.
Notably, plants exposed to mites with higher expression of Tet3 and Tet4 exhibited stronger defense responses, including increased calcium-ion influx, higher generation of reactive oxygen species, and elevated expression of a defensive gene named PR1. The individual application of Tet3 and Tet4 to plants had different effects on plant defense responses, highlighting the specificity of each elicitor’s role. “Taken together, our findings show that these tetranins respond to variable host cues that may optimize herbivore fitness by altering the anti-mite response of the host plant,” remarks Prof. Arimura.
The implications of these findings are twofold. First, understanding the molecular mechanisms that underlie interactions between organisms leads to a better understanding of evolution, ecosystems, and biodiversity. Elicitors such as tetranins act as crucial links in these complex systems, making their detailed study essential for uncovering broader biological insights. From an agricultural perspective, tetranins and similar elicitors offer potential for crop improvement, as insights into the elicitor-sensing system can aid in breeding more sensitive and resilient crops. “Elicitors may be useful as biostimulants that can increase the potential pest resistance of plants,” highlights Prof. Arimura. “The development of such organic farming techniques is extremely meaningful in today’s world, as the environmental and ecological impact of heavy pesticide use grows more severe. Hopefully, identifying elicitors secreted by pests and elucidating their functions will lead to unprecedented spider mite countermeasures.”
With continued research, this fascinating topic could contribute to more sustainable agriculture and enhanced food safety.









.jpeg)