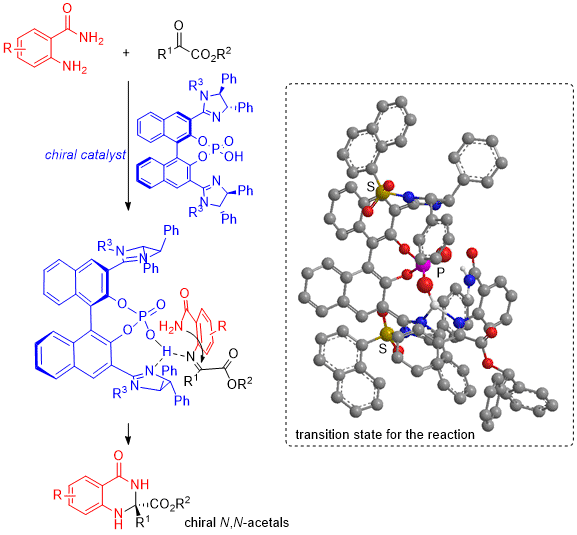
Chiral N,N-acetals are an important component of several bioactive drugs and medicines. Owing to this, chemical reactions that lead to high-purity yield of the desired “enantiomeric” form are highly sought after. In a new study, scientists from Japan demonstrate high selectivity formation of N,N-acetals from reactions between 2-aminobenzamide and various diketones in presence of bis(imidazoline)-phosphoric acid catalyst and look into the possible mechanism, opening doors to facile synthesis of novel pharmaceutical drugs.
In the case of N,N-acetals, several studies have demonstrated their enantioselective preparation from aldehydes, aldimines, or enamines. However, in all these cases, their reaction partner has been limited to aldehyde or imines. While ketones have been employed, with instances of successful enantioselective N,N-acetal synthesis, their use—in general—is not considered effective.
In a recent
study published in Advanced Synthesis & Catalysis, scientists from
Nagoya Institute of Technology (NITech) and
Osaka University in Japan explored this situation with an enantioselective synthesis of chiral N,N-acetals from α-dicarbonyl compounds—compounds with two carbonyl (ketone) groups on the α-carbon—in presence of chiral imidazoline phosphoric acid catalysts, and obtained yields as high as 99% with a maximum enantiopurity of 96%.
“Our study presents the first highly stereoselective formation of chiral N,N-acetals from α-ketoesters using an original catalyst that can be used for other stereoselective synthetic reactions as well,” says Prof. Shuichi Nakamura from NITech, who led the study.
The scientists began by examining the reaction of 2-aminobenzamide with various α-ketoesters in presence of different catalysts. The α-ketoesters differed from one another in the nature of the functional group attached to the α-carbon, while the catalysts chosen were bis(imidazoline)-phosphoric acid with different substituents attached to the nitrogen in the imidazoline ring, mono-imidazoline-phosphoric acid, and two commercial chiral phosphoric acids.
Among these various combinations, scientists found the best yield (99%) and enantiopurity (92%) in the case of α-ketoester with benzhydryl group and a bis(imidazoline)-phosphoric acid catalyst with 1-naphthalenesulfonyl group.
Scientists next examined the reaction of various aminobenzamides (bearing either an electron-donating methyl group or electron-withdrawing fluoro, chloro, and bromo groups) with different α-ketoesters (containing the same benzhydryl group but different functional groups in place of an earlier phenyl group) keeping the same bis(imidazoline)-phosphoric acid catalyst with 1-naphthalenesulfonyl group. All the combinations showed good yield (77–95%) along with high enantioselectivity (82–96%).
In addition, the team looked at the enantioselective synthesis of N,N-acetals via the reaction of N-benzyl isatin (a cyclic α-ketoamide), benzil (an acyclic diketone), and benzaldehyde with 2-aminobenzamide for the same catalyst. All three reactions yielded products with high enantiopurity (91–93%).
The team also proposed a possible mechanism for the N,N-acetal formation reaction corresponding to the best yield (99%), involving a ketimine intermediate with an amide group that helps avoid steric repulsion between the phenyl group on imidazoline, enabling the formation of an (R)-isomer with high enantiopurity.
While the mechanism is still speculative and requires further investigation
, scientists are excited about the potential implications of the experimental results.
“Our new method will enable the synthesis of candidate pharmaceutical drugs that are currently difficult to synthesize, and can even potentially help create and provide people with new and better medicines in the future,” concludes Prof. Nakamura.