In addition to prolonging lifespan, scientists also have long been in pursuit of maintaining human healthspan. For the elderly, as their declined muscle mass and strength cause physical inconvenience, maintaining the health of skeletal muscle is therefore of vital importance so as to keep the ideal quality of life.
Professor Yi-Fan Chen and Professor Yun Yen from
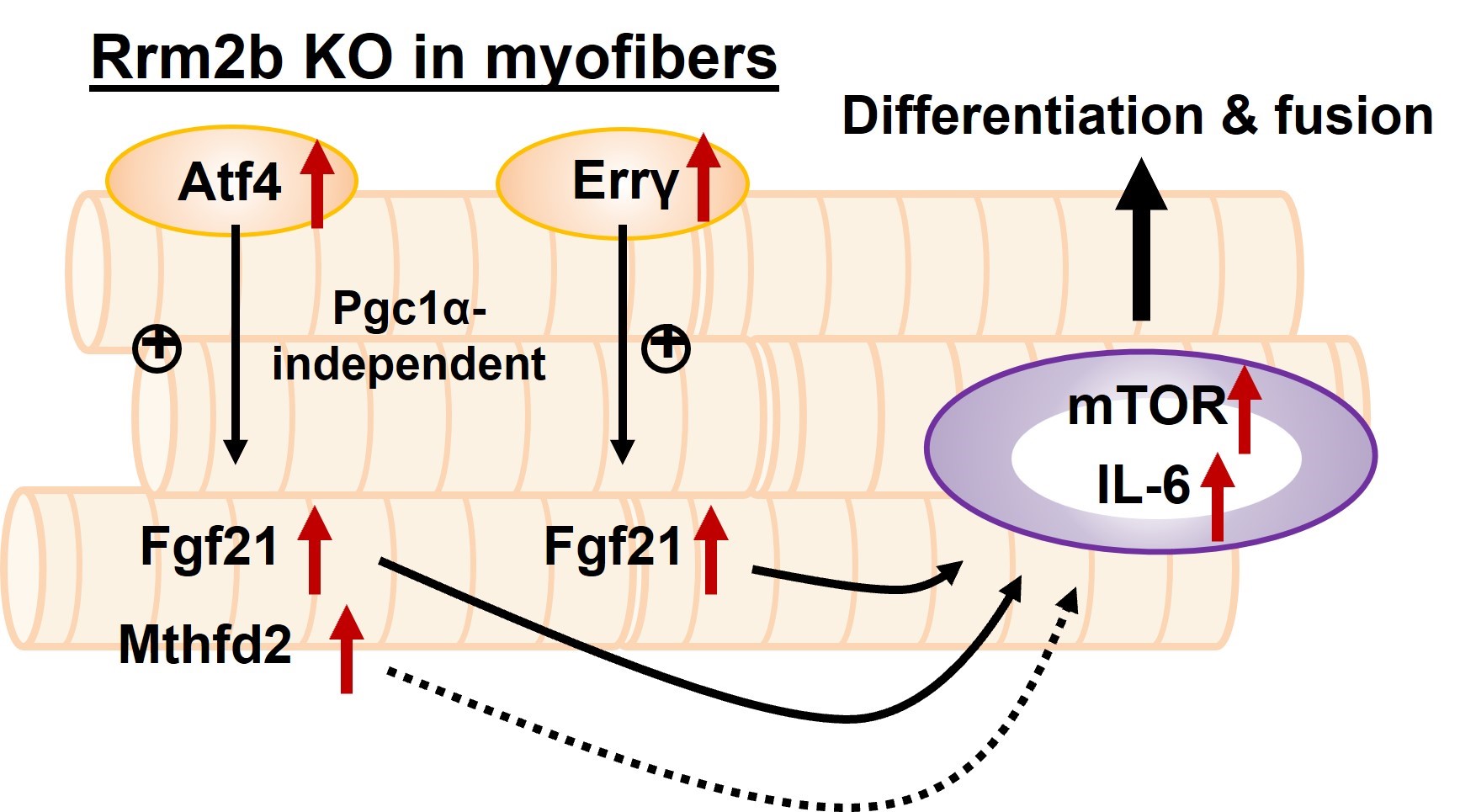
Taipei Medical University, in collaboration with researchers from Japan and Taiwan, have recently published their work in npj regenerative medicine. The research article examines how Ribonucleotide reductase M2B (Rrm2b) modulates the fate of stem cells in skeletal muscle in response to injury. The homeostasis of skeletal muscle relies on the interplay between the muscle stem cells (MuSCs) and their microenvironment (niche). By genetically modified mouse models, Chen unveiled that specific knockout of Rrm2b in the myofibers (a part of niche), but not in MuSCs, led to the weakness of muscles, including loss of muscle mass and strength. These Rrm2b myofiber-specific knockout mice displayed compromised regenerative capacity of muscle with thinner fiber sizes and weaker functioning. Moreover, the lack of Rrm2b in the myofibers resulted in mitochondrial defects, showing a part of the typical characteristics of mitochondrial myopathy.
Furthermore, Chen’s team collaborated with Dr. I-Hsuan Lin, her fellow TMU researcher, for RNA-sequencing to identify several myokines released from Rrm2b-deleted myofibers. These myokines, including FGF-21, GDF-15, and Mthfd2, triggered MuSCs differentiation rather than reentry of quiescence to repopulate the stem cell pool. The decreased MuSC pool due to the imbalance between differentiation and self-renewal of MuSCs thus contributed to muscle weakness and impaired regenerative capacity.
In conclusion, Chen’s study identified a novel role of Rrm2b in muscle homeostasis. Rrm2b in the myofibers plays a critical role in modulating the stem cell fate of MuSCs by an alternation of the microenvironment (niche), and it provides an opportunity for strategy development to treat muscle disorders. Animals with defective Rrm2b expression can probably serve as a disease model for investigating mitochondrial myopathy in mammals. It is expected that such promising research findings will lead to clinical use in promoting muscle health in the coming years.
DOI: https://doi.org/10.1038/s41536-022-00231-w