Further context
This article is a repost from News-Medical, covering a recent study led the research team of Professor Shih-Min Hsia at Taipei Medical University (TMU)’s College of Nutrition. The study investigates the potential effects of sucralose on male reproductive health. The research has also been featured by New York Post and MSN, highlighting the growing international attention to TMU’s contributions to preventive and translational medicine.
Male infertility is a global health concern, impacting 8% to 12% of couples and contributing to nearly half of infertility cases worldwide. Male infertility is affected by hormonal, environmental, and genetic factors that impede spermatogenesis and reproductive function. Dietary and lifestyle changes, including the elevated intake of non-nutritive sweeteners (NNSs) and sugar-sweetened beverages, are among these factors implicated in the growing prevalence of infertility.
Sucralose, an NNS, constitutes 30% of the sweetener market in the United States. Although sucralose has antibacterial properties and lower calories, there are emerging concerns about potential health risks and environmental persistence. It is also a persistent contaminant in aquatic systems, with studies revealing its consistent presence throughout the urban water cycle. Notably, the study highlights concerns about sucralose-6-acetate, a genotoxic byproduct of sucralose manufacturing and metabolism, which may exacerbate health and environmental risks. Despite research on NNSs, data on potential links between sucralose and male infertility are limited.
About the study
In the present study, researchers evaluated the effects of sucralose on male reproductive health. Male Sprague-Dawley (SD) rats, aged six weeks, were acclimated for a week under controlled conditions and subsequently randomized to one of four experimental groups. Sucralose was administered at 1.5 mg/kg, 15 mg/kg, 45 mg/kg, or 90 mg/kg for two months.
The controls received deionized water. Body weight was monitored weekly for eight weeks. At the end of the study, animals were euthanized, and blood samples were collected for biochemical analyses. Organs such as the liver, spleen, heart, testes, epididymis, and kidneys were harvested for histopathological evaluation.
The cauda epididymis was cut into pieces and briefly maintained in culture medium, and the supernatant was used for sperm motility analysis. Sperm samples were subject to a Western blot analysis to evaluate DNA damage markers. Besides, enzyme-linked immunosorbent assay was used to measure follicle-stimulating hormone (FSH), luteinizing hormone (LH), and Kisspeptin1 (KISS1).
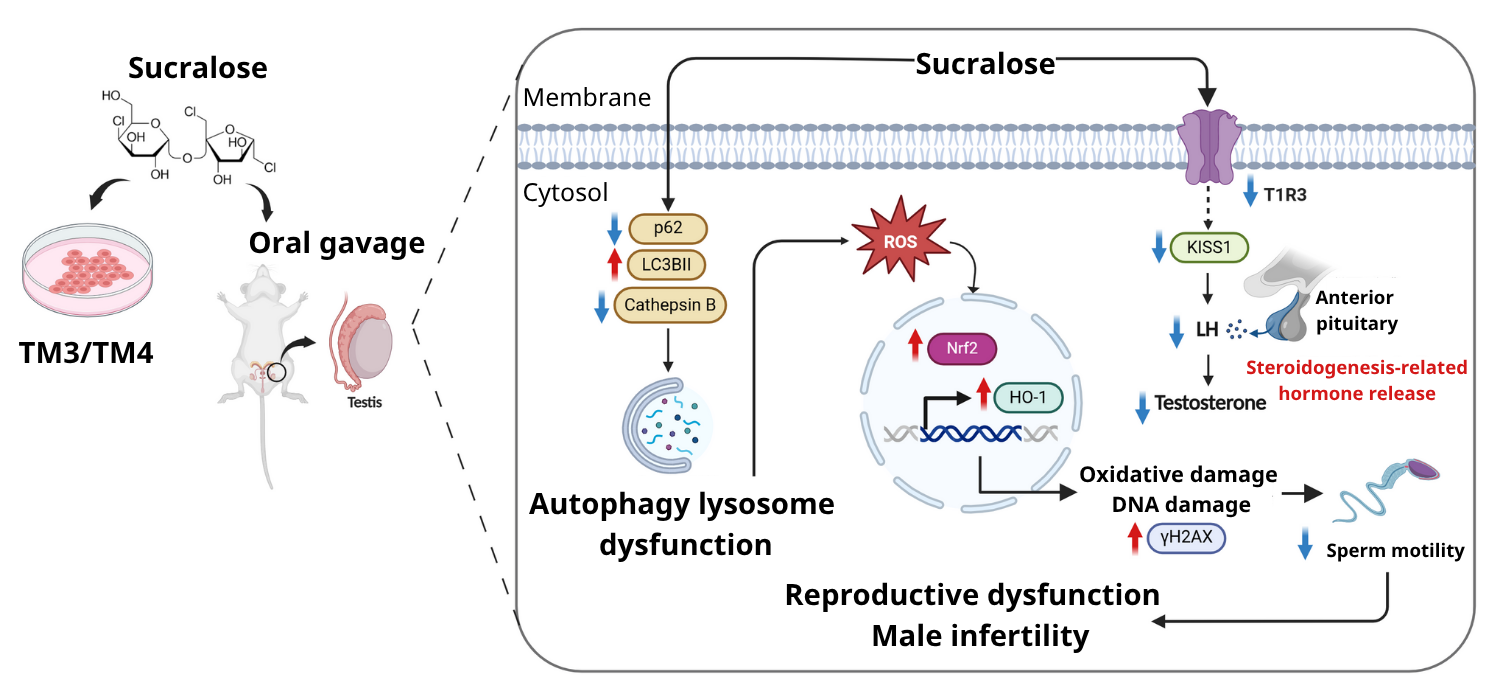
Chemiluminescence immunoassays were performed to measure serum testosterone, alanine aminotransferase (ALT), and aspartate aminotransferase (AST). Further, mouse Sertoli cells (TM4) and Leydig cells (TM3) were treated with varying concentrations of sucralose for 24–72 hours. These cells were subject to intracellular reactive oxygen species (ROS), cell viability, and Western blot analyses. To assess autophagy-lysosome dysfunction, researchers used Bafilomycin A1, a compound that blocks lysosomal acidification, revealing impaired fusion of autophagosomes and lysosomes.
The Kolmogorov-Smirnov test assessed data normality. Non-parametric tests were applied for data violating normality assumptions. A two-way analysis of variance was performed to evaluate the effects of sucralose and exposure duration. Group differences were compared using the Mann-Whitney U test or the Student’s t-test.
Findings
TM3 and TM4 cells exposed to varying sucralose concentrations (1 μM, 10 μM, 100 μM, 1000 μM, and 10,000 μM) had significantly lower cell viability. Cells also showed higher levels of microtubule-associated protein 1A/1B light chain 3B, form II (LC3B-II) at 1000 or 10,000 μM and slightly lower p62 levels. These changes, combined with reduced cathepsin B (a lysosomal enzyme), suggest impaired autophagic degradation. Following sucralose treatment, there was a significantly lower expression of cathepsin B, indicative of impaired lysosomal function.
ROS levels in TM3 and TM4 cells after sucralose exposure at 1 mM, 2.5 mM, 5 mM, 7.5 mM, or 10 mM were significantly higher; sucralose-treated cells also had elevated nuclear factor erythroid 2-related factor 2 (Nrf2) expression and heme-oxygenase 1 (HO-1) levels, suggesting an increase in oxidative stress. Notably, exposed cells had a reduction in taste receptor type 1 member 3 (T1R3) protein expression.
Moreover, co-treatment with a known T1R3 antagonist (lactisole) repressed T1R3 expression more than sucralose treatment alone. To further examine the relevance of T1R3 modulation, rat pituitary adenoma cells (RC-4B/C) were treated with sucralose, with or without lactisole co-treatment. This revealed a significant reduction in LH levels dose-dependently. Lactisole co-treatment exacerbated this suppression, especially at lower sucralose levels.
SD rats exposed to sucralose showed no differences in body weight, AST or ALT levels, and heart and liver indices between groups. Although the appearance of reproductive organs was not remarkably different, exposed animals had significantly lower epididymis and testis indices. Further, rats showed significant reductions in serum testosterone and LH levels and serum and testicular KISS1 levels. KISS1, a key regulator of the hypothalamic-pituitary-gonadal (HPG) axis, is critical for initiating puberty and maintaining reproductive hormone balance; its suppression may directly contribute to impaired fertility.
Sucralose exposure also reduced protein levels of T1R3 in the testes. Exposed animals had abnormal sperm morphology (with coiled and bent tails) and lower sperm viability. Histological examination of the testes showed changes in the seminiferous epithelium, including severe vacuolization, disrupted germ cell organization, and nuclear condensation.
DNA damage was also observed in sperm, indicative of cellular impairment. The testes of sucralose-exposed animals had higher levels of LC3B and lower levels of p62, suggesting changes in autophagy. Moreover, exposed animals had higher serum and testicular levels of malondialdehyde, indicating increased lipid peroxidation.
Conclusions
Taken together, sucralose exposure adversely affects male reproductive outcomes in rats by inducing oxidative stress, causing DNA damage, and disrupting autophagy. The study notes that in vitro doses (up to 10 mM) likely exceed typical human dietary exposure, warranting caution in extrapolating results to real-world intake levels.
The findings underscore the need for careful evaluation of dietary NNSs and call for better food safety regulations to alleviate potential risks. Additionally, the environmental persistence of sucralose and its byproduct, sucralose-6-acetate, highlights broader ecological concerns.
Further studies are required to examine dose-response relationships, long-term effects, and underlying molecular mechanisms to comprehensively delineate the adverse effects of sucralose.
Look for More Information
Original Study: Exposure to Sucralose and Its Effects on Testicular Damage and Male Infertility: Insights into Oxidative Stress and Autophagy
News: Sucralose disrupts male fertility by damaging sperm and altering hormones in animal study